As the medical landscape evolves, exosome-based therapies emerge as a revolutionary approach to treatment, captivating researchers and clinicians alike. These nano-sized vesicles, secreted by cells, carry genetic material and proteins that can influence neighboring cells, presenting a promising avenue for targeted therapies.
However, the integration of quality assurance in biomanufacturing processes remains a significant hurdle in realizing the full potential of exosome therapies. Regulatory complexities, combined with the need for stringent quality control measures, underscored the importance of establishing robust frameworks to ensure the safety and efficacy of these innovative agents.
This article explores the critical aspects of integrating quality assurance into the biomanufacturing process of exosome-based therapies, highlighting the advantages of exosomes, the challenges faced in production, and the strategies necessary for compliance and standardization within a rapidly advancing field.
Advantages of Exosomes as Therapeutic Agents
Exosomes are small vesicles secreted by various cell types, such as immune and mesenchymal stem cells. They play a vital role in therapeutic applications due to several unique advantages.
Advantages of Exosomes:
- Efficient Drug Delivery:
Exosomes can carry active substances, such as nucleic acids and proteins, directly to target cells. Their natural origin allows better acceptance by the body compared to synthetic drug carriers. - Natural Intercellular Communication:
They enhance intercellular communications, which is essential for therapeutic potential in various clinical applications. - Versatility:
Derived exosomes can be tailored to transfer specific therapeutic agents. This versatility makes them suitable for numerous conditions. - Low Immunogenicity:
Exosomes have low risk of triggering immune response, making them ideal for repeated administrations. - Specificity:
Exosomes can be derived from specific parental cells, allowing targeted treatment by carrying precise signals.
Overall, the potential of exosomes in therapies is highlighted by these characteristics, promising safer and more effective modes of treatment delivery compared to traditional methods. Limitations like exosome biogenesis and isolation methods, however, are areas for further research.
Regulatory Challenges in Exosome Production
Producing exosomes for therapeutic use involves several regulatory challenges. Exosomes come from human cells and must be safe and effective. Regulatory bodies set guidelines to ensure consistency and quality. These guidelines cover all stages, from isolation methods to final product testing.
This process is strict because exosomes are used in clinical studies with human patients. Meeting these regulations can be complex and time-consuming. Yet, it is vital for the success of any exosome-based therapy.
Standards for Quality Control
Quality control is crucial in exosome production. It’s important to monitor size distribution, active substances, and purity levels. Various methods, such as size-exclusion chromatography, help achieve these standards.
Quality control processes must ensure exosome products meet high standards. The aspects to check include:
- Size Distribution: Ensures consistent therapeutic potential and efficacy.
- Purity Levels: Indicates the absence of unwanted contaminants.
- Biological Activity: Confirms that the exosomes’ mechanism of action is effective.
Consistency in these areas guarantees that therapies are both safe and effective.
Compliance with Regulatory Frameworks
Compliance with regulatory frameworks ensures that exosome therapies are ready for clinical applications. Different countries have their own rules. So, producers must navigate these to market exosome products.
Key compliance components include:
- Documentation: Ensure complete records of the production process.
- Testing Protocols: Follow standardized testing for quality assurance.
- Batch Consistency: Ensure uniformity across different production batches.
Adhering to these frameworks is crucial for the successful delivery of exosome-based therapies in global markets. Regulatory bodies review these aspects before approving clinical and therapeutic applications. Proper adherence can enhance the acceptance and success rate of exosome therapies.
Methods for EV Isolation and Characterization
Extracellular vesicles (EVs) hold great promise in therapeutic applications. Quality control in their production is crucial. EVs, like mesenchymal stem cell-derived exosomes, have therapeutic potential in various clinical studies. The steps in isolating and characterizing EVs can influence these outcomes. The techniques used ensure that EVs are safe and effective for cell therapies.
Standard Techniques for Isolation
EV isolation is the first step in ensuring high-quality products. Common methods include ultracentrifugation, size exclusion chromatography (SEC), and filtration. Ultracentrifugation separates EVs based on their size and density. It is one of the most popular methods but can be harsh on the vesicles. Size exclusion chromatography, on the other hand, uses a gel column to separate particles based on size. SEC is gentle and reduces the loss of active substances. Another method is filtration, which uses membrane filters to separate EVs from other components. Each method has its strengths and weaknesses, impacting the EVs’ size distribution and quality.
Characterization Methods
Characterizing EVs is crucial for understanding their mechanism of action and therapeutic potential. Common characterization techniques include nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), and flow cytometry.
NTA measures the size and concentration of EVs by tracking their Brownian motion. It is effective for assessing size distribution. DLS also analyzes particle size but works well for larger vesicle populations. Meanwhile, flow cytometry identifies and sorts EVs based on surface markers. This method is especially useful when isolating specific cell types, like immune cells or stromal cells.
A comprehensive understanding of EV characteristics ensures the right therapeutic applications and enhances their performance in clinical and preclinical studies. Thus, combining different characterization methods provides a full profile of the EVs’ capabilities.
- Nanoparticle Tracking Analysis (NTA): Measures size and counts EVs in a sample.
- Dynamic Light Scattering (DLS): Gives insights into larger vesicle populations.
- Flow Cytometry: Sorts EVs by surface proteins, identifying parent cell types.

In summary, high-quality therapeutic EV production relies on careful isolation and characterization. Selecting the right methods ensures optimal intercellular communications and effective drug delivery systems. Quality control at every step is vital to successful clinical applications.
Quality Management Tools in EV Research
Ensuring high-quality control in therapeutic extracellular vesicle (EV) production is crucial. EVs have therapeutic potential due to their roles in nucleic acids and drug delivery systems. Quality management tools help improve consistency and reliability in this field. They are vital in controlling variables like size distribution and active substances to maintain the therapeutic potential of EVs.
Role of Standardization
Standardization in EV research ensures that methods and results are comparable across different studies. By using standardized isolation methods, like size-exclusion chromatography, we can achieve more reliable results. This also aids in the characterization of EVs from Mesenchymal Stem Cells and other cell types. Standardization is key for future clinical applications of EVs, ensuring consistency in exosome products and therapeutic applications.
Reproducibility in Experimental Results
Reproducibility is essential for scientific credibility. In EV research, this means producing the same results consistently in preclinical studies and clinical studies. Reproducibility can be improved by using clear protocols for EV isolation and analysis. Consistent exosome biogenesis and secretion conditions help achieve reliable data. By focusing on reproducibility, researchers can better understand the mechanism of action in EV therapies.
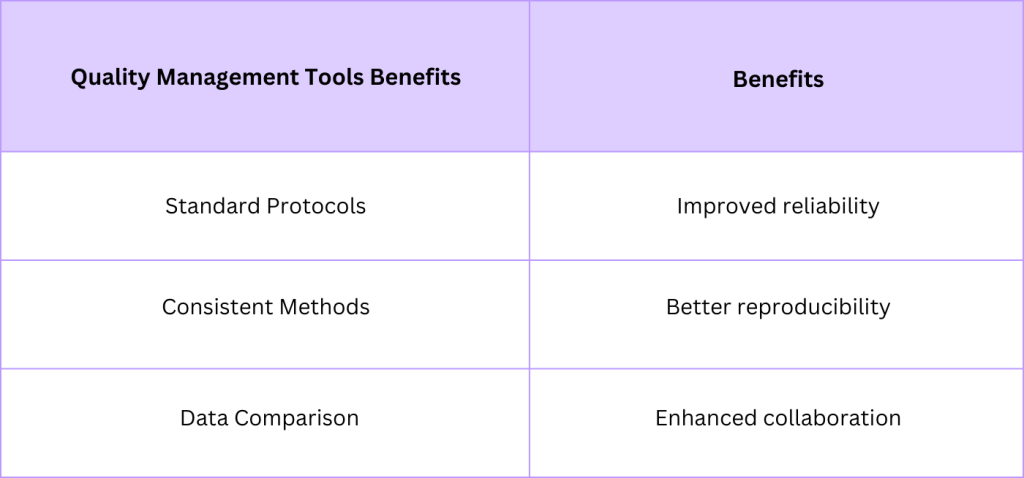
In summary, quality control in EV research requires robust tools and practices to ensure reliable and reproducible results. With proper standardization and focus on reproducibility, the therapeutic applications of EVs will continue to grow.
Developing Potency and Safety Assays
Quality control in therapeutic EV production is vital. To ensure their efficacy and safety, developing precise potency and safety assays is crucial. These assays evaluate the therapeutic potential of extracellular vesicles (EVs), like those derived from mesenchymal stem cells and other parental cell types. Potency assays measure the active substances in EVs, while safety assays check for harmful impurities. For instance, tests ensure nucleic acids are correctly packaged. These steps guarantee EVs are effective for clinical applications like cell therapies and drug delivery systems. Through rigorous testing, we understand the mechanism of action and ensure their safe use.
Importance of Rigorous Testing
Rigorous testing in EV production is essential for safe clinical studies. Testing identifies and eliminates inconsistencies in size distribution and isolation methods. By maintaining strict standards, we ensure EVs have the correct therapeutic applications. This process involves evaluating the exosome secretion and biogenesis from human cells. Precision in testing helps avoid potential risks in therapeutic and preclinical studies. Moreover, it ensures the stability of exosome products and their reliability in drug delivery systems.
Assay Validation Processes
Assay validation guarantees the accuracy and reliability of results. It ensures that methods like size-exclusion chromatography and size exclusion chromatography are effective. During validation, each assay undergoes thorough checks to confirm its precision, sensitivity, and specificity. This process involves testing the reproducibility and stability of assays over time. Validation provides confidence in the therapeutic potential of EVs and their safe integration into clinical settings. By following strict validation guidelines, position papers and clinical studies can rely on these assays to ensure EV safety and potency.
Cross-Disciplinary Collaboration in EV Research
Extracellular Vesicles (EVs) hold great promise in medicine. They are used in clinical applications to treat various diseases. Research in this field requires teamwork across different disciplines. Combining expertise is crucial for unlocking the therapeutic potential of EVs.
Importance of Teamwork among Disciplines
Teamwork is vital for advancing EV research. Scientists, clinicians, and engineers must work together. Each discipline brings unique skills. Biologists understand the mechanism of action and how EVs interact with cells. Chemists focus on isolation methods and size distribution. Engineers help optimize drug delivery systems and ensure proper exosome quality. Working collaboratively increases the chances of success in preclinical studies and clinical studies.
Sharing Knowledge and Resources
Sharing knowledge and resources is essential in EV research. Different researchers can provide insights on parental cells, immune cells, and stromal cells. Sharing advanced techniques like size-exclusion chromatography enriches the research field. Cross-disciplinary teams can effectively share data on nucleic acids and human cells. This boosts the development of therapies and enhances intercellular communications. Effective collaboration leads to better therapeutic applications and innovative cell therapies.
Cross-Disciplinary Collaboration in EV Research
Extracellular Vesicles (EVs) hold great promise in medicine. They are used in clinical applications to treat various diseases. Research in this field requires teamwork across different disciplines. Combining expertise is crucial for unlocking the therapeutic potential of EVs.
Importance of Teamwork among Disciplines
Teamwork is vital for advancing EV research. Scientists, clinicians, and engineers must work together. Each discipline brings unique skills. Biologists understand the mechanism of action and how EVs interact with cells. Chemists focus on isolation methods and size distribution. Engineers help optimize drug delivery systems and ensure proper exosome quality. Working collaboratively increases the chances of success in preclinical studies and clinical studies.
Sharing Knowledge and Resources
Sharing knowledge and resources is essential in EV research. Different researchers can provide insights on parental cells, immune cells, and stromal cells. Sharing advanced techniques like size-exclusion chromatography enriches the research field. Cross-disciplinary teams can effectively share data on nucleic acids and human cells. This boosts the development of therapies and enhances intercellular communications. Effective collaboration leads to better therapeutic applications and innovative cell therapies.
Role of Preclinical and Clinical Studies
Preclinical and clinical studies are crucial in developing exosome-based therapies. They help understand the safety and effectiveness of exosomes for therapeutic applications. Scientists first use preclinical studies to test exosomes in lab settings, often with cell types like immune cells and Mesenchymal Stem Cells (MSCs). These studies focus on how exosomes interact with human cells and their potential to carry nucleic acids as active substances. This phase is where the mechanism of action and exosome quality are examined. Afterward, clinical studies follow, where exosome products are tested in humans.
Phases of Clinical Research for Exosomes
Clinical research for exosomes progresses through specific phases.
- Phase 1: This phase evaluates safety. It involves a small group of volunteer participants. The goal is to assess any adverse effects of the therapy.
- Phase 2: In this phase, scientists study effectiveness. They also gather more data on safety. A larger group of people participates to see if the exosome-derived treatment works as expected.
- Phase 3: This phase involves an even larger population. The purpose is to confirm effectiveness, monitor side effects, and compare the treatment to commonly used therapies.
- Phase 4: After approval, post-market studies are done. These monitor the long-term effects and benefits of the exosome therapy.
Relevance of Animal Models in Efficacy Assessments
Animal models are vital in evaluating the efficacy of exosome therapies before human trials. They help predict how exosomes will behave in the body and how they affect different cell types. For example, Mesenchymal Stem Cell-derived exosomes can be tested on animals to learn about their therapeutic potential. These models help researchers understand the size distribution of exosomes and optimize the isolation method, like size-exclusion chromatography. Moreover, animal studies play a role in drug delivery systems by demonstrating how exosomes facilitate intercellular communications. Preclinical studies using these models are essential for a comprehensive assessment before moving on to clinical applications.
Emerging Regulatory Frameworks
The field of therapeutic extracellular vesicles (EVs) is expanding rapidly. As these novel treatments move towards clinical applications, clear regulatory frameworks are needed. It’s important to ensure safe and consistent production of EV products. Regulating bodies worldwide are working on standardizing the process. These frameworks will help guarantee the quality of EV-based therapies. They also aim to protect patients by setting strict guidelines for production and testing.
Regulations in Japan
Japan is proactive in setting rules for therapeutic EV production. The Pharmaceuticals and Medical Devices Agency (PMDA) is taking the lead. They focus on quality control, especially in isolation methods and size distribution. In Japan, EVs derived from mesenchymal stem cells and other cell types are of particular interest. The PMDA’s framework covers testing procedures and classification of cell therapies. Japan’s approach ensures that therapeutic potential is met with high standards of safety.
Regulations in South Korea
South Korea is also advancing in regulating therapeutic EVs. The Ministry of Food and Drug Safety (MFDS) oversees this area. Their focus is on nucleic acids, exosome biogenesis, and exosome secretion. South Korean regulations emphasize the mechanism of action and intercellular communication. The country is also exploring size exclusion chromatography for quality control testing. This method helps achieve consistent size distribution in exosome products. South Korea’s regulatory steps are vital for advancing clinical and preclinical studies.